Reference: Langmuir 2011, 27, 10061-10071
Title: Encapsulation of Active Cytoskeletal Protein Networks in Cell-Size Liposomes
Authors: F.-C. Tsai, B. Stuhrmann and G. H. Koenderink
Review by: Antoine Diguet
Title: Encapsulation of Active Cytoskeletal Protein Networks in Cell-Size Liposomes
Authors: F.-C. Tsai, B. Stuhrmann and G. H. Koenderink
Review by: Antoine Diguet
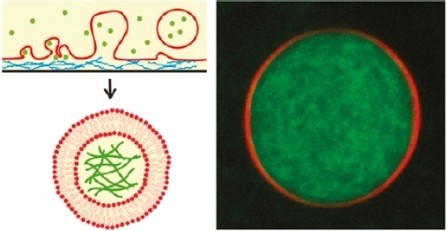
This article demonstrates that cytoskeletal actin-myosin networks can be encapsulated in giant liposomes by hydration of lipids in an agarose gel. Here, two major conditions have to be respected to preserve the biological activity of the cytoskeletal systems into giant liposomes: maintain a high salt level (physiological buffer) and have a fast procedure (< 1 h).
According to authors, all of existing giant liposome formation procedures have at least one drawback:
- electroformation [1] method is slow (3 h), requires low salt concentrations and the encapsulation yield is small
- microfluidic inkjetting technique [2] requires specialized equipment
- inverted emulsion method [3] requires multiple steps
- electroformation [1] method is slow (3 h), requires low salt concentrations and the encapsulation yield is small
- microfluidic inkjetting technique [2] requires specialized equipment
- inverted emulsion method [3] requires multiple steps
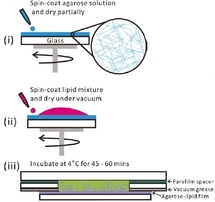
The proposed method is facile, quick and reproducible. It is derived from the work of Horger et al. [4], where giant liposomes have been obtained by gentle hydration of an agarose hydrogel film infiltrated with lipids. It consists in three stages, depicted in the Figure on the right. A uniform layer of gelling agarose is spin-coated on a glass slide (i). Then a lipid solution is spin-coated on the agarose layer and dried (ii). Finally, the film is hydrated in a buffer containing actin and optionally myosin (iii).
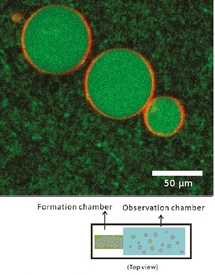
After 1 h of welling, unilamellar liposomes with an average diameter of about 20 µm are obtained (Figure). After heating at room temperature, actin filaments can be visible and form an entangled network inside the liposome. In the external medium, filaments can be digested by adding an enzyme (DNaseI). The encapsulation yield can be increased by adding 5 mol% of PEGylated lipids in the lipid film. Moreover, the encapsulated networks can be selectively anchored to the inner leaflet of the membrane via biotin-streptavidin linkages.
Even if it is clear that agarose layer, PEGylated lipids and spin-coating are necessary for a successful experiment, the exact swelling mechanism remains unclear.
To conclude, this work is interesting for two main reasons:
On the one hand, these protein-filled liposomes represent useful systems for in vitro study of interactions between actin networks and membrane and of consequences on cell shape and mechanics.
On the other hand, this work is a new strategy for an easy encapsulation of biological macromolecules in cell-sized liposomes (could it be possible with DNA?).
On the one hand, these protein-filled liposomes represent useful systems for in vitro study of interactions between actin networks and membrane and of consequences on cell shape and mechanics.
On the other hand, this work is a new strategy for an easy encapsulation of biological macromolecules in cell-sized liposomes (could it be possible with DNA?).
References
[1] M. I. Angelova and D. S. Dimitrov, Faraday Discuss. Chem. Soc. 1986, 81, 303-311
[2] J. C. Stachowiak, D. L. Richmond, T. H. Li, A. P. Liu, S. H. Parekh and D. A. Fletcher, Proc. Natl. Acad. Sci. USA 2008, 105, 4697-4702
[3] A. Yamada, T. Yamanaka, T. Hamada, M. Hase, K. Yoshikawa, D. Baigl, Langmuir 2006, 22, 9824-9828
[4] K. S. Horger, D. J. Estes, R. Capone and M. Mayer, J. Am. Chem. Soc. 2009, 131, 1810-1819
[1] M. I. Angelova and D. S. Dimitrov, Faraday Discuss. Chem. Soc. 1986, 81, 303-311
[2] J. C. Stachowiak, D. L. Richmond, T. H. Li, A. P. Liu, S. H. Parekh and D. A. Fletcher, Proc. Natl. Acad. Sci. USA 2008, 105, 4697-4702
[3] A. Yamada, T. Yamanaka, T. Hamada, M. Hase, K. Yoshikawa, D. Baigl, Langmuir 2006, 22, 9824-9828
[4] K. S. Horger, D. J. Estes, R. Capone and M. Mayer, J. Am. Chem. Soc. 2009, 131, 1810-1819